Host selection
In search of the ideal host for Pr3+ ions
Pr3+-based phosphors are a large group of materials, where the host lattice plays an important role in how the material behaves. These host materials can include compounds like fluorides, chlorides, phosphates, silicates, borates, and others. However, not all of them allow visible-to-UVC upconversion to occur. Because of this, choosing the right host for Pr3+ ions is a key step in making efficient upconverting materials.
First of all, the host determines the energy of emitted radiation in the UV range. Because the 5d electrons are not shielded from the environment like the 4f electrons, the configuration of the 4f5d levels in the crystal field of ligands is significantly impacted by the crystal structure and host material composition. The energy of the lowest, emitting 5d level of Ln3+ ions is determined by the parameter called redshift (D(A)), which is the difference between the energy of the first 4f → 4f5d transition for free Pr3+ ions and those incorporated into host A. The magnitude of D(A) depends on various parameters, including the ligand type, coordination number, polyhedron type, and the average distance between Ln3+ and ligand atoms [1]. It could be calculated according to the formula:
D(A) = E5d(free) - E5d(A) (1)
where, E5d(free) is the position of the lowest 5 level of RE3+ as a free ion, and E5d(A) likewise for RE3+ ion doped into compound A (see Figure 1). To obtain emission within the range of 200 – 280 nm, the value of E5d(A) should be between 36,960 – 51,250 cm-1 (taking into account a Stokes shift of approximately 2500 cm-1).
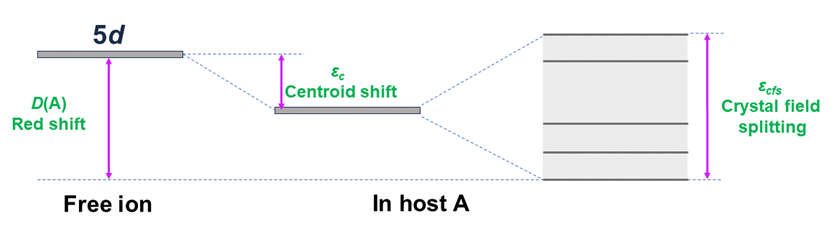
Figure 1. Schematic illustration of crystal field splitting of 5d levels in octahedral field.
However, in the case of Pr3+ions, E5d(A) cannot be higher than the energy of the 1S0 level, which is usually around 47,000 cm-1. Otherwise, instead of broadband 4f5d → 4f emission, strong narrow bands corresponding to 1S0 → 3HJ, 3FJ, 1D2, 1G4, 1I6, and 3P0 → to 3HJ, 3FJ transitions will be observed. This phenomenon is called ‘photon cascade emission’ (PCE) or ‘quantum cutting’ (QC) (see Figure 2). To avoid quenching of 4f5d → 4f emission by the PCE process, E5d(A) of Pr3+ should not be higher than 46,000 cm-1. Consequently, hosts that provide appropriate splitting of 5d orbital must be characterized by D(A) between 15,580 cm-1 and 24,680 cm-1.
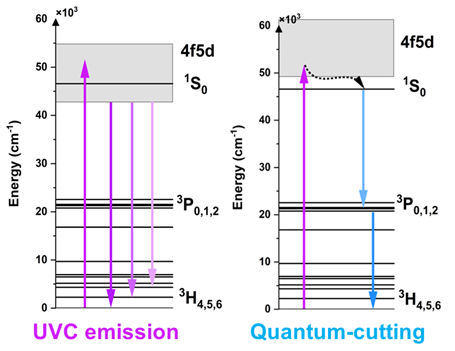
Figure 2. Energy level diagrams presenting luminescence processes under VUV excitation for Pr3+ ions with different energies of the lowest 5d level.
Peter Dorenbos collected and summarized information about crystal field splitting of Ln3+ 5d levels for over 300 compounds [2]. According to his study, fluorides, which stand out from low phonon energies, have small values of D(A), meaning they are more suitable for the PCE process rather than broadband UVC emitters. Although chlorides or bromides exhibit a larger redshift of 5d levels, they are very often hygroscopic, and by absorbing water, their luminescence can be quenched. On the other side, oxide matrices, due to the huge variety of materials including simple and complex oxides, are characterized by a wide range of D(A) values (10 000 – 30 000 cm-1). The above properties combined with high physical and chemical stability make Pr3+-doped oxides, such as phosphates, silicates, and borates, the most convenient materials for UVC light sources.
In addition to affecting the energy of the 5d configuration, the host also significantly influences the efficiency of visible-to-UVC upconversion. The first key factor is the bandgap energy (Eg) between the valence and conduction bands in the matrix. For effective UVC emission, the 5d levels of Pr3+ ions must be energetically below the conduction band edge to prevent energy loss via ionization. The simplified scheme of the ionization process is presented in Figure 3A. Another influential factor is the Stokes shift (ΔS), the energy difference between absorption/excitation and emission bands. ΔS affects the probability of non-radiative relaxation of 5d states via thermally induced crossover quenching: when ΔS is large (Figure 3B, left), quenching is efficient, favoring visible emission. When ΔS is small (Figure 3B, right), quenching occurs only at high temperatures, allowing most energy to be emitted as UVC radiation. This suggests that host compounds inducing a small ΔS are optimal for blue-to-UVC upconversion with Pr3+.
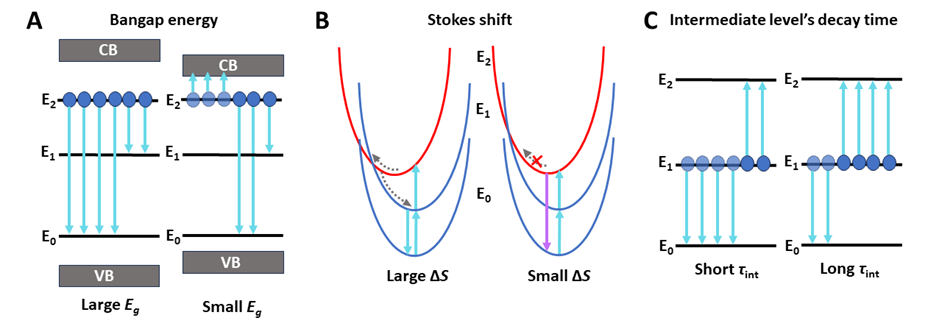
Figure 2. Schematic presentations of factors influencing the efficiency of the Vis-to-UVC upconversion process. (A) The bandgap energy between the valence (VB) and conduction (CB) bands. (B) The decay time of the intermediate level (τint). (C) Stokes shift (ΔS) of UVC emission.
The decay time of the intermediate level (τint) is another crucial parameter influencing UC luminescence. Unlike other factors, τint is directly linked to the upconversion process itself. Generally, a longer τint increases the probability of second-photon absorption, thus enhancing UC process efficiency (see Figure 3C). Most studies identify the 3P0 level as an intermediate state in upconversion. Consequently, the conventional approach to enhancing Vis-to-UVC UC has been to increase the 3P0 excited state decay times by selecting host crystals with lower phonon energies. In such hosts, multiphonon relaxation (MPR) from the 3P0 to the lower 1D2 level and phonon-assisted cross-relaxation (CR) [3P0, 3H4 → 1D2, 3H6] processes are reduced, leading to an extended 3P0 lifetime (see the ESA 1 or ETU 1 mechanism in Figure 4). This approach was applied by Cates et al. [3] in Lu7O6F9:Pr3+, which showed a higher UVC UC luminescence compared to Y2SiO5:Pr3+. Despite some improvements, gains in UC efficiency have been lower than expected, leading to further investigation of the 1D2 level as an intermediate. This level can be effectively fed in a high-phonon energy host by MPR and CR processes (see the ESA 2 or ETU 2 mechanisms in Figure 4). Because the 1D2 level has a significantly longer decay time than the 3P0 level, the enhancement of UC luminescence can be observed, which was subsequently demonstrated in β-Y2Si2O7:Pr3+ [4]. Our recent studies also further supported this strategy. We developed a high-phonon Sr3(BO3)2:Pr3+ phosphor with efficient 3P0 → 1D2 MPR, achieving a significant increase in UC luminescence compared to Y2SiO5:Pr3+ [5]. These findings open up promising potential for high-phonon energy compounds in efficient visible-to-UVC upconversion, which have so far been underestimated.
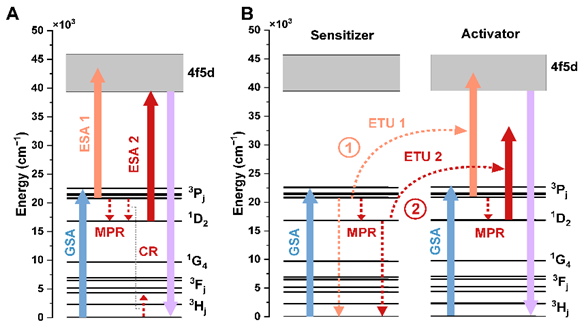
Figure 4. Schemes of possible upconversion mechanisms: (A) excited state absorption (ESA) and (B) energy transfer upconversion (ETU) observed for low- (1) and high-phonon energy (2) materials. For simplicity, the CR processes were omitted in the ETU mechanism.
Bibliography:
[1] Dorenbos, P. 5d-Level Energies of Ce3+ and the Crystalline Environment. I. Fluoride Compounds. Phys Rev B 2000, 62, 15640–15649, doi:https://doi.org/10.1103/PhysRevB.62.15640.
[2] Dorenbos, P. The 5d Level Positions of the Trivalent Lanthanides in Inorganic Compounds. J Lumin 2000, 91, 155–176, doi:https://doi.org/10.1016/S0022-2313(00)00229-5.
[3] E. L. Cates, A. P. Wilkinson, J.-H. Kim, Visible-to-UVC upconversion efficiency and mechanisms of Lu7O6F9:Pr3+ and Y2SiO5:Pr3+ ceramics, J Lumin 2015, 160, 202, doi: https://doi.org/10.1016/j.jlumin.2014.11.049.
[4] E. L. Cates, F. Li, Balancing intermediate state decay rates for efficient Pr3+ visible-to-UVC upconversion: the case of β-Y2Si2O7:Pr3+, RSC Adv 2016, 6, 22791, doi: https://doi.org/10.1039/C6RA01121G.
[5] P. Zdeb, N. Rebrova, P. J. Dereń, Discovering the Potential of High Phonon Energy Hosts in the Field of Visible-to-Ultraviolet C Upconversion, J Phys Chem Lett 2024, 15, 9356. doi:https://doi.org/10.1021/acs.jpclett.4c02053.