Upconversion
Step towards innovative sources of UVC radiation
In conventional luminescence materials, the energy of the excitation photon must be higher than the energy of the emitted photon, which is called Stokes emission. In the case of the UVC light generation (200 – 280 nm), a high excitation energy (100-200 nm) is required to excite the activator ion. This is a serious practical limitation of using Ln3+-based materials as UVC radiation sources because excitation devices needed for this purpose are usually large, expensive, and require vacuum conditions.
Upconversion particles are an excellent solution to this limitation because they can be excited with radiation of lower photon energy than the energy of the emitted photons [1]. Due to that upconverting nanoparticles are attractive materials for many applications such as bioimaging, temperature sensing, solar cells, lighting, and displays. Pr3+ ions are particularly interesting upconverting activators since they can absorb two photons of blue light and convert them into one photon of ultraviolet radiation. The two most common mechanisms leading to visible-to-UVC upconversion are depicted in Figure 1. The left scheme presents energy transfer upconversion (ETU). Two nonexcited Pr3+ ions participate in the process: sensitizer (Pr(I)) and activator (Pr(II)). First, both are excited from the ground 3H4 level to the 3P2 level and relax non-radiatively to the 3P0 level. Then, energy transfer (ET) occurs between them, leading to the excitation of the activator to the 4f15d1 configuration and the relaxation of the sensitizer to the ground state. On the other side, in the excited state absorption (ESA, right scheme) only one Pr3+ ion is required. The excitation of 4f15d1 levels is reached by absorbing the 444 nm photon by the ion, which is already excited to the 3P0 level.
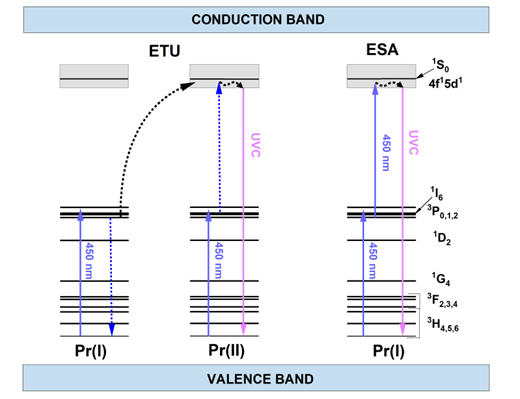
Figure 1. Schemes of possible upconversion mechanisms: ETU (on the left) and ESA (on the right). Solid arrows represent the radiative transitions, while dotted arrows correspond with the nonradiative ones.
The possibility of excitation of UVC upconversion by blue light about 450 nm is convenient from a practical point of view. Since Nakamura, Amano, and Akasaki revolutionized LED lighting, with the invention of efficient blue light-emitting diodes, 450 nm LEDs became cheap and commercially available sources of blue light. Looking for other excitation sources, we could not ignore the largest and most accessible visible radiation source, which is the sunlight. The solar spectrum at the Earth's surface exhibits maximum irradiance in the blue-green range. Moreover, polychromatic is another advantage of solar radiation. Due to the presence of multiple Stark levels of Pr3+ ions, the ESA process can include the summing of energy of different wavelength radiation. Cates et al.[2] demonstrated UVC luminescence of Y2SiO5:Pr3+ samples by excitation them with a combination of two excitation sources: 488 nm + 515 nm and 447 nm + 589 nm. In both cases, intense UVC emission was observed. A polychromatic excitation mechanism was also proposed by Wu and co-workers [3] who detected UVC luminescence for LiYF4 samples irradiated by sunlight.
The idea of converting solar radiation into germicidal UVC light is being developed for the creation of self-cleaning surfaces. Such systems would not require any chemical or UV lamps for disinfection, but only exposure to the sun. Pr3+-doped materials, such as ortho-borates, seem to be excellent candidates for this purpose. Although the quantum efficiency of the upconversion process is usually around 1%, these materials are expected to emit a weak intensity of UVC luminescence, which we believe could be an advantageous feature. Due to the high germicidal effectiveness of UVC light and continuous exposure to sunlight, efficient disinfection should be obtained while ensuring human safety.
Bibliography:
[1] Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem Rev 2004, 104, 139–173, doi: https://doi.org/10.1021/cr020357g.
[2] Cates, E.L.; Kim, J.-H. Upconversion under Polychromatic Excitation: Y2SiO5:Pr3+, Li+ Converts Violet, Cyan, Green, and Yellow Light into UVC. Opt Mater 2013, 35, 2347-2351, doi:https://doi.org/10.1016/j.optmat.2013.06.030.
[3] Wu, J.; Zheng, H.; Liu, X.; Han, B.; Wei, J.; Yang, Y. UVC Upconversion Material under Sunlight Excitation: LiYF4: Pr3+. Opt Lett 2016, 41, 792–795, doi:https://doi.org/10.1364/OL.41.000792.